Information on Spectrophotometry / Spectrophotometry
A major player in spectrophotometry is the absorption of light which allows for an easy qualitative and quantitative analysis.
So in undertaking spectrophotometry, the use of a spectrophotometer is required, as it is built with both a spectrometer and a photometer. Other components of a spectrophotometer include a light source (monochromator), a cuvette and a light detector, and data analysis software.
Application of Spectrophotometer
-Chemistry
-biochemistry (for enzyme-catalyzed reactions)
-Physics
- Biology
-clinical studies
Testing of water quality has been made easy through a spectrophotometer. How safe water is for drinking, how pure and clear it is, what properties are contained in it, etc. All these can be tested quickly and cheaply through a spectrophotometer.
Before drugs are released to society for usage, it needs to go through some rigorous testing to ensure that it works for the purpose for which it is created. A spectrophotometer has proven to be a cost and time-saving way of doing this.

Types of Spectrophotometer
There are two main types of Spectrophotometer, namely the Single beam Spectrophotometer and the Double beam Spectrophotometer. These two Spectrophotometers can undertake different types of analysis and do not come with the same specifications.
The single-beam Spectrophotometer
The single-beam Spectrophotometer is designed to give light through a sample by providing one beam. This spectrophotometer is designed for more detailed analysis and is preferred to provide a higher dynamic rate. Their design is also compact, which means they can easily be moved around.
The Double Beam Spectrophotometer
This type of Spectrophotometer is designed with precision in mind, as it can release dual beams, which would undertake special functions to help arrive at a more definite and precise report. So, in this case, their automation is more seamless due to their dual procedural setting.
More types:
Fluorescence spectrophotometer
-Atomic absorption spectrophotometer.
-Micro Spectrophotometer
-Visible Spectrophotometer
-UV-VIS spectrophotometer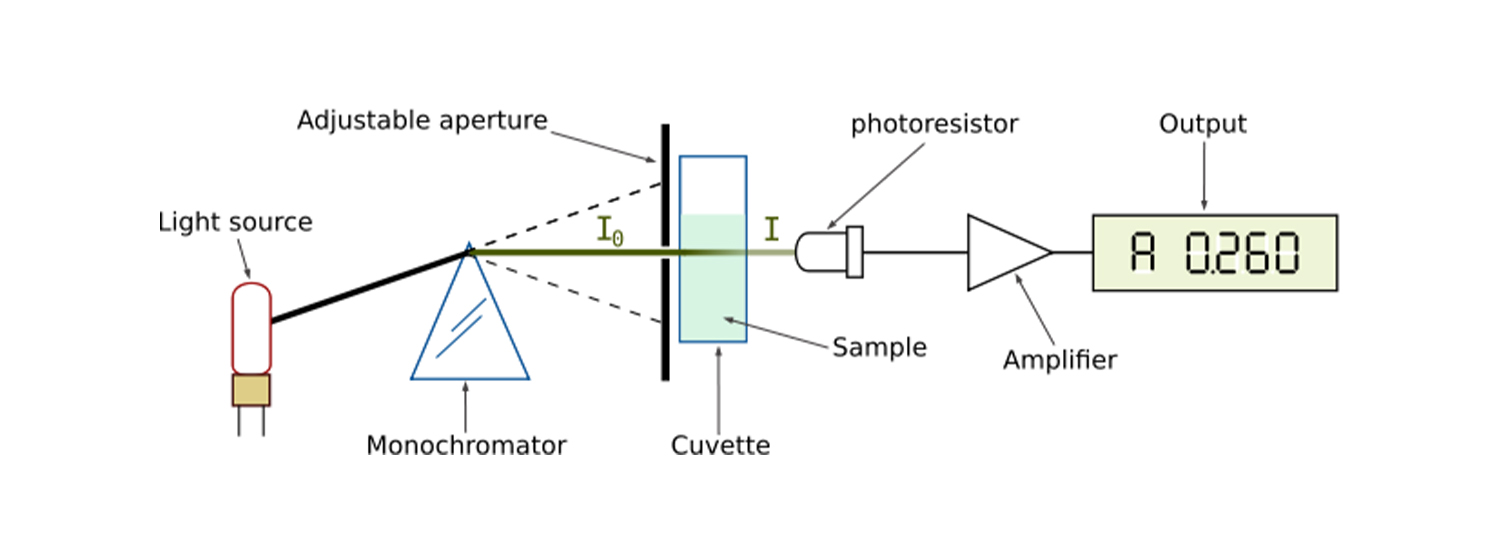
Level of absorbance and transmittance
The user can control the temperature of a Serology Laboratory Water Bath by using a digital or analogue interface. A light will usually indicate that the water bath is working, and once the correct temperature has been reached the water bath will turn on and off to maintain the constant temperature. Certain Laboratory Water Baths have a safety setting preventing the water from heating to a higher temperature.
Different types of Laboratory Water Baths also exist, for example shaking water baths, which are used to mix substances together and have additional controls allowing users to control the speed and frequency of movements. Laboratory Water Baths do not have to contain water and can use alternate fluids such as oil depending on the required temperature and viscosityIn undertaking its process, the spectrophotometer needs to cognizance the level of absorbance and transmittance of the solution. So when light passes through, the level of absorbance and transmittance would determine the process the scientist would need to take to make their analysis accurate. So this is why a cuvette is used for the sample container, to know how well it would absorb light.
However, the transmittance of the process needs to be calculated using the following equation:
Transmittance (T) = It/I0
It = Light intensity after passing the cuvette (transmitted light)
I0 = Light intensity before passing the cuvette (incident light)
Absorbance (A) = – log10 T = – log IS/IR
Also, Absorbance can be measured with this equation, combining both the Beer-Lambert Law and Spectrophotometer: A = ƐCL.
A = absorbance of light at a specific wavelength
Ɛ = molar extinction coefficient (the absorbance of 1 mole of a substance dissolved in 1-liter solvent)
C = the molar concentration of a sample
L = the optical path length of a sample.
Measuring Absorption with a Spectrophotometer
To do this, getting more information on the value of the molar extinction coefficient, optical path length, and molar concentration are required.
Molar Extinction Coefficient – Ɛ is the value at which light at a given wavelength accentuates with the chemical solution.
The SI unit is m2/mol but sometimes expressed as M-1 cm-1 or L mol-1 cm-1. The molar extinction coefficient can also be derived from literature sources in libraries and online.

