Laboratory Guidelines for the Detection of Monkeypox Virus Infection
The World Health Organization (WHO) Director-General declared the escalating global monkeypox outbreak a Public Health Emergency of International Concern (PHEIC) last July 23, 2022. According to WHO, there are more than 35,000 cases across 92 countries/territories, and 12 deaths as of August 17. Most reported cases (with travel history) traveled to areas in Europe and North America.
Monkeypox is a rare disease caused by infection with the monkeypox virus (MPXV)—an enveloped double-stranded DNA virus that belongs to the same family of viruses that cause smallpox. This virus can be transmitted via contact with bodily fluids, lesions on the skin, or internal mucosal surfaces with symptoms like fever, headache, and muscle aches. The incubation period of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days (about three weeks).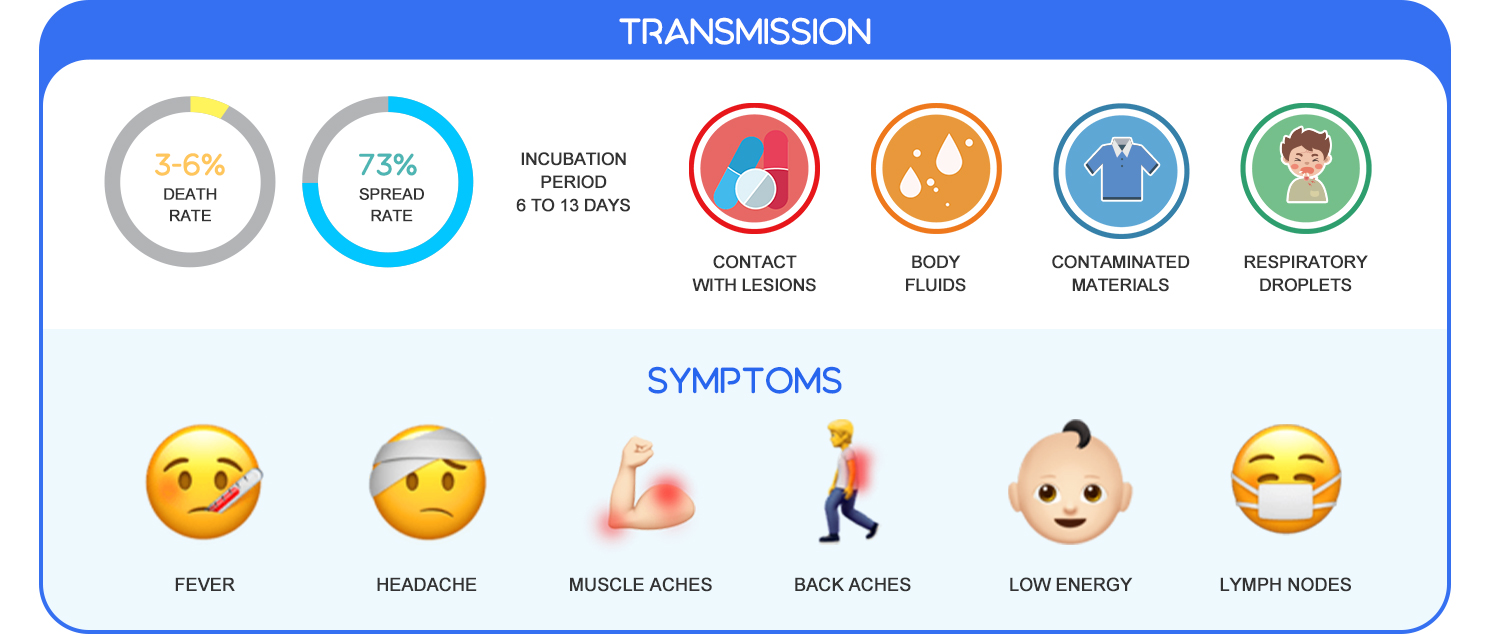 According to the WHO, the best diagnostic specimens are directly from the rash—skin, fluid, or crusts, or biopsy where viable. Other methods like antigen and antibody detection may not be useful as they do not distinguish between orthopoxviruses.
According to the WHO, the best diagnostic specimens are directly from the rash—skin, fluid, or crusts, or biopsy where viable. Other methods like antigen and antibody detection may not be useful as they do not distinguish between orthopoxviruses.
A risk-based approach is recommended in manipulating the specimens from the suspected, probable, or confirmed cases of monkeypox in the laboratory. Therefore, the procedures for detecting the presence of the MPXV, which is categorized as a risk group 3 pathogen, must be carried out in a Biosafety Level 2 laboratory using the list of equipment shown below.

Equipment Guide for Monkeypox Virus Detection
Specimen and Reagent Storage
The MPXV specimens are recommended for refrigeration at 2°C to 8°C or must be frozen (-20°C or lower) within an hour of collection. If the transport time exceeds seven days, specimens should be stored at -20°C or below. Meanwhile, -70°C is advised for long-term specimen storage (>60 days from collection).
Biobase Laboratory Refrigerators, Laboratory Freezers, and Ultra-low Temperature Freezers are equipped with well-designed refrigeration systems and high-quality materials suitable for storage of temperature-sensitive biological samples that require critical storage conditions.

Sample Handling and Preparation
MPXV can be transmitted during the specimen processing stage due to contaminated material or improper methods. As a result, increased biosafety measures are to be observed. Prior to sample inactivation, specimens from patients with suspected MPXV must be prepared in a functional Class II biosafety cabinet; however, this is not required for properly inactivated specimens.
Aside from the protection that the biosafety cabinets provide, Biobase Class II Biological Safety Cabinets are NSF 49 and EN 12469 compliant with user-friendly features and are coated with antimicrobial powder on all its external and internal painted surfaces for improved safety. Centrifuges are necessary for the separation of the specimen's liquid and solid components. Safety cups or sealed rotors are advised if essential for the procedure.

Detection and Analysis
Nucleic acid amplification testing (NAAT) is conducted to confirm the MPXV infection. To detect unique viral DNA sequences, either PCR real-time or conventional polymerase chain reaction (PCR) is used. There are two steps involved in virus detection. The first PCR detects orthopoxviruses but does not identify the species while the second PCR (or sequencing) specifically detects MPXV.
A real-time PCR thermal cycler provides efficient amplification of nucleic acids for virus analysis. Biobase offers the new Swift™ PCR Thermal Cyclers with a built-in computer for stand-alone operation from PCR program input, monitoring, and analysis.
